Recently, Zhang Jinlong, a professor of the School of Chemistry of our university, and Zhou Liang, an Associate Professor of the School of Resource and Environmental Engineering, cooperated with their team to publish their research achievements titled as Peroxymonosulfate Activation via Non-contact Electron Transfer Process (NCETP) for Efficient Organic Pollutant Removal in the top periodical in the field of environment, Water Research. In the study, a new catalytic mechanism, non-contact electron transfer process, was proposed. The mechanism shows an extremely high efficiency of pollutant degradation and selectivity in Fenton-like reactions, providing a brand-new idea for the development of efficient water treatment technology.
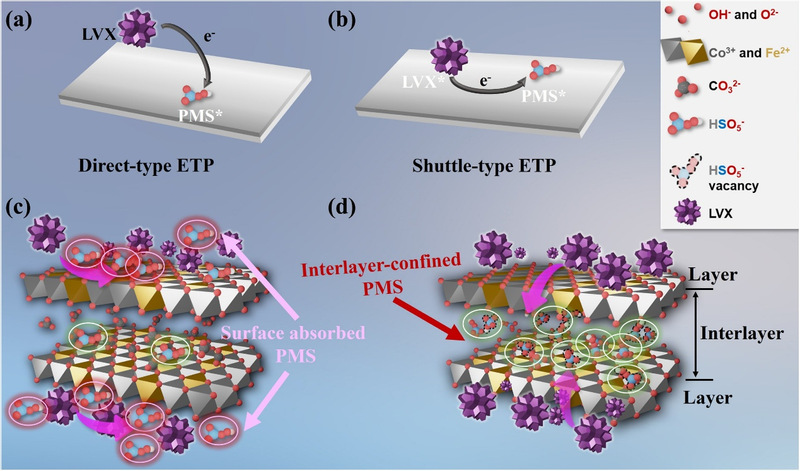
Residual antibiotics in water, such as levofloxacin (LVX), etc., have been a global environmental problem. Although advanced oxidation processes based on sulfate-free radicals can effectively degrade these pollutants, the traditional path of free radicals faces problems, including a short service life, a high possibility of being interfered with by water quality, a low rate of oxidant utilization, etc. As a non-free-radical path, the process of electron transfer has strengths, including high selectivity, strong anti-interference performance, etc., but it still faces multiple challenges in actual application, such as a low efficiency of electron delivery, too many side reactions, etc. The study first put forward the non-contact electron transfer process (NCETP) in the system of layered double hydroxides/peroxymonosulfate (LDH/PMS). Different from the traditional process of electron transfer, NCETP realizes the physical isolation of pollutants on both sides, namely oxidants and catalysts. PMS is confined between different layers of LDH, while LVX and other macromolecular pollutants are absorbed on the surface of the catalysts. The two parts can't directly contact each other. Researchers ingeniously adopted the memory effect and the strategy of ion exchange to successfully produced the CoFe LDH catalyst (CLHSO) with HSO₅⁻ vacancies, thus making it absorb and anchor itself between different layers of PMS. The microenvironment in the field between layers not only improved the local concentration of PMS, but also realized efficient electron delivery based on a hydrogen-bridge structure. The experiment showed that the number of transferred electrons during the NCETP process was 2.58 times higher than that of traditional ETP. The efficiency of LVX degradation within 30 minutes exceeded 95%, and the system was still efficient and stable in a wide range of pH values (5-11) and a complicated condition of water quality. The team constructed a continuous flow reactor with polyester fiber loaded with CLHSO as fillers to verify the actual application potential of the technology. During the continuous operation for as long as 24 hours, the system's LVX degradation rate was kept higher than 99%, and the metal leaching concentration was far lower than the national emission standard. In addition, after treating the continuous flow, the chromaticity of dyeing wastewater was significantly lowered. The toxicity assessment result showed that the ecological toxicity of the degraded product was significantly lower than originally researched LVX, and the treated solution had an effect of total colon bacillus inactivation, showing proper environmental friendliness.
In the paper, the East China University of Science and Technology was the only correspondence unit, and Pei Wenkai and Hao Chenchen, two doctoral students of the School of Resource and Environmental Engineering, were both first authors. The corresponding authors were Professor Zhang Jinlong and Professor Zhou Liang. Paper writing was also thoughtfully guided by Professor Liu Yongdi and Associate Professor Lei Juying. The study was supported by the National Key Research and Development Program of China, the National Natural Science Foundation of China, and other programs.
Link to the original article:
https://www.sciencedirect.com/science/article/pii/S0043135425016999#fig0001




